How Often Should GMP Controlled Temperature Units (CTUs) be Requalified?
How often should GMP CTUs be requalified? This is not a simple question to answer. Determining the appropriate requalification interval and testing...
3 min read
Alcami Validation Team : Nov 13, 2023 10:09:28 AM
In the biopharmaceutical industry, thermal validation is required for sterilizers. Sterilizers are used in cleanrooms for equipment used in manufacturing, medical devices, steam-in-place, and more. Thermal validation helps ensure that the quality of the equipment is up to par with pharmaceutical safety standards. These standards ensure that the devices and drugs being manufactured are safe and effective for patients.
When equipment is not correctly sterilized, there is a risk of contamination, which can potentially impact the end quality of the product. That’s why thermal validation is such an essential part of the manufacturing process. Different types of thermal validation processes can be used, depending on which materials are being validated and what temperature they are required to maintain. In addition to thermal validation, it is also important to look at microbial challenges when looking at accumulated lethality.
Accumulated lethality and its importance
The standard temperature for sterilization is 121°C. However, not all materials can be sterilized at that temperature due to heat sensitivity. When that happens, the sterilization time must be adjusted so the material can be sterilized to the same degree as would happen at 121°C in one minute. That adjusted value is called accumulated lethality (AF0).
Accumulated lethality (AF0) can be defined as the exposure time (in minutes) of an object for sterilization at a specific temperature, and it’s crucial in calculating the accurate sterilization temperature correctly. The FDA recognizes accumulated lethality
(AF0) as a substitute form of methodology for determining the success of a completed thermal sterilization process.
Accumulated lethality (AF0) is calculated using data from thermal mapping studies executed during sterilization cycles. The calculation is then used to determine passing or failing results identified in a validation protocol.
Sterility Assurance Level vs. Log Reduction
The requirements defined in a validation protocol to test sterility capabilities are based on evaluating multiple components. In addition to the general site and industry requirements, those components include Sterility Assurance Level (SAL) and Log Reduction.
SAL is quite different from Log Reduction. However, both are used together to identify sterility capabilities. SAL identifies the chance of survival for each spore in a population after the sterilization process, whereas Log Reduction refers to the total amount that a population is reduced by.
Industry-standard for Sterility Assurance Level is 10-6, which means there is a 1 in 1,000,000 chance of an organism surviving the sterilization process. 10-6 is the lowest probability of spore survival, so medical devices and surgical equipment typically aim for that level. However, it is important to note that the CDC says that level was “strictly arbitrary and not associated with any adverse outcomes (e.g., patient infections).”
A differing value of 10-3 can be approved for certain materials that have varying properties. For example, clothing that patients wear during surgery and similar materials may have different SAL levels. Because contamination is a significant concern for patients with compromised immune systems or other high-risk conditions, pharmaceutical companies must consider SAL.
Log Reduction is the elimination of a pathogenic bioburden. Log stands for logarithm, a mathematical term that means a quantity representing the power to which a fixed number must be raised to produce a given number. A log reduction takes the power in the opposite direction. In the validation process, log reduction demonstrates how valuable and practical the sterilization device is, showing the potential of the sterilization process. It also gives manufacturers an idea of how impactful the sterilization process is on the product.
Log Reduction Example
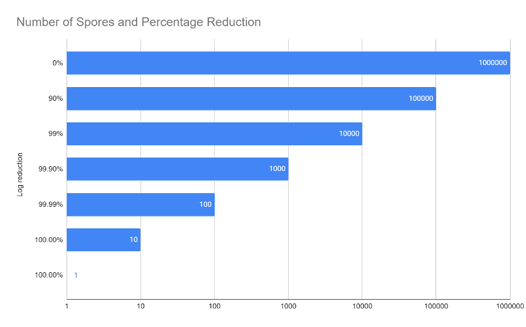
Assume that you have a bacterial colony where the population is 1 million spores. A one log reduction (1x10-1) in population is the same as reducing a population by 90%, so you would be left with 100,000 spores in your colony. A two log reduction (1x10-2) in population reduces that same population by another 90%; this would be a total reduction of 99% from the original population count. Thus, you would be left with 10,000 spores in your colony. The below charts show the population at various log reductions.
| Log Reduction | Number of Spores | Percentage Reduction |
| 0 | 1,000,000 | 0% |
| 1 | 100,000 | 90% |
| 2 | 10,000 | 99% |
| 3 | 1,000 | 99.9% |
| 4 | 100 | 99.99% |
| 5 | 10 | 100% |
| 6 | 1 | 100% |
If you apply the one-in-a-million chance that a single spore survives, it would mean executing another 1x10-6 log reduction to the existing 1x10-6 reduced colony. Therefore, in a bacterial colony where the population is 1 million spores, achieving a SAL of 1x10-6 requires a 12 log population reduction. A 12 log population reduction is industry standard and a requirement in most thermal validation facilities. This means there is a 0.000001% (or one in a million) chance of any individual spore from the initial population surviving the sterilization process.
Accumulated lethality (AF0) can be used along with the D121 value of a microorganism to calculate the log reduction in the micro-organism population. The D121 value is defined as the number of minutes at 121°C required to attain a 1 log population reduction for that microorganism. For example, achieving a 6 log population reduction of a microorganism with a D121 value of 1.0 would re-quire a sterilization cycle that collects an AF0 of 6 minutes. If the D121 value were increased to 2.0, the sterilization cycle would need to collect an AF0 of 12 minutes.
Conclusion
Both Sterility Assurance Level and Log Reduction play a significant role in the thermal validation process, which is why it’s crucial to ensure that technicians incorporate and understand both. The thermal validation process may be complex, but it is an integral part of life sciences manufacturing. At Alcami, we take thermal validation seriously. We understand that without thermal validation, the entire supply chain could suffer dire consequences. Without thermal validation, pathogenic bioburdens could contaminate material or equipment causing delays in patients receiving critical medical devices or drugs. When you want the highest possible standards in thermal validation, give Alcami a call.
To learn more about our validation services, visit our validation solutions webpage today.

How often should GMP CTUs be requalified? This is not a simple question to answer. Determining the appropriate requalification interval and testing...

Why Summer Mapping? Thermal mapping of warehouse space is an important step in control of the supply chain for life science companies throughout the...